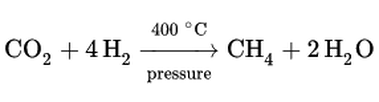
Author: Paul Zilberman MDAnaesthesiologist, Israel MOTTO: I was born in 1960. As a child I was thrilled to witness the first man in space, as per stories, in those years a direct TV transmission was still a dream. And even if it had been possible, I was one year of age, so… But later on, I was able to see the launch of the Apollo missions and the common US-Soviet programs Soyuz- Apollo. As many other terrestrials I was thrilled to watch, both from distance and close up, those “white pencils” with the painting of “The United States of America” climbing faster and faster, leaving behind a huge ball of fire… Then the first carrying rocket segment detaching and falling back to Earth… I was amazed seeing how only after a short time those “people out there” were floating and smiling, waving their hands and telling us everything is ok. I was reading about the many experiments that were carried out during the flights, I was even able to look now and then at the flight path, little understanding what were the sinusoidal lines appearing on the huge Command Center screen, where so many people were sitting in front of the computers with the microphones and earpieces connected. I didn’t understand then, exactly, why so many people were dealing with so few in space. Well, time went on, Skylab appeared, then the ISS, the shuttles…wow…all in a lifetime. As time went on and understanding grew, on top of my medical school and, later on, anaesthesia residency, other questions arose: how do the astronauts eat, drink, wash, use the toilets? And many other daily mundane things we take for granted down here. Modern medicine is all about numbers. Not in the dry sense of how many patients we see or haw many beds a hospital has; that’s the headache of the medical administrators. In my education “numbers” are pressures, concentrations, volumes etc. One question I was asking myself for a long time was how the astronauts breathe in those confined spaces for such long periods of time. After all you have several people in a closed artificial environment. Exactly like in the submarines, but with a critical difference. A submarine can produce its needed oxygen by water hydrolysis; and usually there’s plenty of it in the oceans. In case of problems a submarine can surface, problem solved. Not so in space. And here come into play those numbers: how much oxygen an astronaut needs, how many astronauts are in that space, how much CO2 is produced, how it is removed, and how is oxygen produced when, think of it, you don’t have it around the spaceship or station. Metabolic issues are studied to the tiniest detail, and experience is gathered with every new mission. In many fields. I would like to bring here only one aspect: the removal of CO2. This text is not intended to explain in detail all the process, but just to make the reader raise a brow and say “I never thought of that”! And open his appetite to search for more. The CO2 we produce is related to many physiological factors. Once exhaled it needs to be taken away from our faces, otherwise we inhale it back, a process known in anaesthesiology (and not only) as “rebreathing”. Think of this specific issue in space where there is no wind to wash away the CO2 from your face. Well, another problem, the need for cabin fans to circulate the air. The removal of CO2 and the whole chemical reactions used in order to clean the closed environment of this gas while retaining the oxygen is energy dependent and follows several chemical steps. The whole process is known as “The Sabatier reaction”, from the name of one of the two French chemists that discovered it in 1897, Paul Sabatier and Jean-Baptiste Sendersen. The moment I got closer to one other way of CO2 removal in space was when I read, post factum, of the almost tragedy of Apollo-13. A faulty contact in the transport module produced an explosion. The astronauts were forced to move quickly to the lunar module but…the CO2 absorbent filters there were not enough to remove enough CO2 in order to keep them alive till return to Earth. Well, you are invited to read the whole story. But on this occasion, I learned about the LiOH based CO2 filters. The chemical reaction is the same as for any -OH based CO2 absorbent used in the anaesthesia machines only that, at least as a theoretical chemical calculation, their absorbent capacity on a CO2 volume/unit of absorbent mass can go up to five times more than what is commonly used in operating rooms (ORs).
The contribution of a special technology that preserves the spatial configuration of the absorbent helps to prevent a mechanical process called “channeling” that further reduces the lifetime of the other absorbents. Recently, with the Li prices going up, most probably due to its use in electric car batteries, the LiOH absorbent became financially non-competitive. However, the technology of maintaining that spatial configuration was kept. It is used today in the production of the same CO2 absorbents but with CaOH as the active substance. The absorbent is manufactured as cartridges, each type fitting a special anaesthesia machine. It may seem like a drawback, but if we consider the longer lifetime, the almost non-existent dust producing issue, the ease of replacing them, by the end of the day it may be a step forward in making our existing ORs more efficient in all respects. All that is happening in the space confined “piece of Earth out there” is in fact human activity and use of resources to the maximum. In anaesthesiology we call it “closed circuit”. The same way we use our experience on Earth to preserve life in space, we can do the reverse, help preserve, or at least improve life here based on the experience gathered from the space missions. It all depends on us. Comments are closed.
|
Welcometo the InnovaSpace Knowledge Station Categories
All
|
InnovaSpace Ltd - Registered in England & Wales - No. 11323249
UK Office: 88 Tideslea Path, London, SE280LZ
Privacy Policy I Terms & Conditions
© 2024 InnovaSpace, All Rights Reserved
UK Office: 88 Tideslea Path, London, SE280LZ
Privacy Policy I Terms & Conditions
© 2024 InnovaSpace, All Rights Reserved



 RSS Feed
RSS Feed