Adriana Bos-MikichDepartment of Morphological Sciences, ICBS, Federal University of Rio Grande do Sul, Brazil 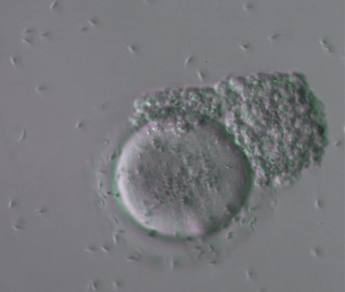 Human sperm and oocyte Image: Nilo Frantz Human Reproduction Centre, Porto Alegre Human sperm and oocyte Image: Nilo Frantz Human Reproduction Centre, Porto Alegre Amazing as it may seem, as the number of spaceflights has increased and life aboard space stations has become a reality, the effects of high levels of space radiation and microgravity (micro-G) on mammalian reproduction are still largely unknown. Therefore, the study of reproduction in space is a very important subject for the future of space missions. Research conducted with experimental non-mammal animals, such as sea urchins, fish, amphibians, and birds has concluded that micro-G does not prevent animal reproduction. However, mammalian reproduction presents specific features, such as ovulation, sperm and oocyte (egg) transit in the reproductive tract, embryo attachment, implantation and placentation, which are specific to mammalian reproduction and cannot be studied in non-mammal species. Surprisingly, little information is available today on how these processes occur in conditions of microgravity. 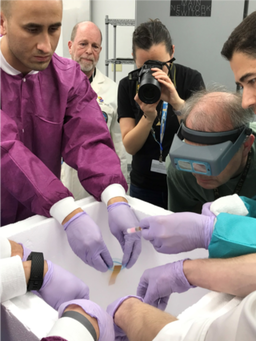 KSC Researchers preparing Micro-11 experiment sperm samples for launch to the ISS. Credits: NASA KSC Researchers preparing Micro-11 experiment sperm samples for launch to the ISS. Credits: NASA A first problem requiring investigation is the supposed difficulty that sperm and egg cells may have to travel along the female reproductive tract to accomplish natural fertilization under micro-G conditions. A project with 100% relevance is NASA’s Micro-11 research, which aims to examine putative motility alterations in human and bull sperm during spaceflight. “Micro-11 provides fundamental data indicating whether successful human reproduction beyond Earth is possible, and whether countermeasures are needed to protect sperm function in space” (NASA). Rapid directional sperm motility is a key factor for successful fertilisation under natural circumstances. Sperm cells need to swim up the uterine cervix, travel through the uterine cavity and fallopian tubes to meet and fertilise the oocyte. To accomplish all these tasks, human sperm respond to chemoattractant signals, to temperature gradient, and to fluid flow. These guidance mechanisms occur naturally along the female reproductive tract and are important for the sperm-egg encounter and for natural fertilisation to occur in the fallopian tubes, but will these mechanisms be sufficient in micro-G? Experimental studies with mice have revealed impaired male germ cell generation under microgravity conditions. Of concern are alterations seen in the physiology of testicular cells observed under conditions of simulated microgravity, which may obscure the starting point of mechanisms that lead to long-lasting tumorigenic processes. However, a recent research has shown that the deleterious effects of microgravity on germ cell proliferation, oxidative metabolism and autophagy may be, at least partially, prevented by the presence of antioxidants in the germ cell culture medium. This finding represents an important contribution to the current knowledge of microgravity effects on germ cell tumour metabolism and development. According to estimates, nearly one in six couples worldwide seek out assisted reproduction technologies for having a child. Thus, it is expected that fertility assistance may also be necessary in space due to naturally occurring fertility problems or due to micro-G induced infertility conditions. A report from the 35th annual meeting of the European Society of Human Reproduction and Embryology recently revealed that frozen human sperm retain their viability and fertilising capacity in outer space, similar to sperm samples stored in liquid nitrogen under Earth’s gravity conditions. This represents a reassuring finding, particularly when considering the importance of sperm cryostorage for fertility preservation, such as in the case of cancer patients who may lose their reproductive potential due to oncological treatments. It also allows us to hypothesise that donor intra-uterine insemination may represent a viable option for having a child under the micro-G conditions found in space stations. The more complex assisted reproduction technology (ART), “in vitro fertilisation” (IVF), developed by Sir Robert Edwards and Dr. Patrick Steptoe, allows fertilisation to occur outside of the body, i.e., outside its natural tubal environment, in a plastic petri dish, giving rise to the term “test-tube” baby. Its main purpose is to promote fertilisation when the natural encounter of sperm and oocyte is not possible, as is the case of women presenting blocked uterine horns. In summary, oocytes are first collected from the ovaries, (performed by transvaginal ovarian puncture and aspiration) and placed in a petri “IVF” dish containing a culture medium that mimics the tubal micro-environment. The male partner produces a sperm sample, which is prepared for mixing with the oocytes, after which the IVF dish is maintained in a warm incubator (37oC) in a laboratory for fertilisation to take place. The sperm must be able to swim and penetrate the oocyte membrane for fertilisation to be accomplished, and to assist this they are placed close together to facilitate their interaction and fusion. The resulting embryos remain in the incubator for up to six or seven days, before being transferred to the womb. However, fertilisation will not occur under natural or even IVF conditions when the ejaculated sperm do not present rapid directional motility in a condition called asthenospermia, a common cause of unsuccessful reproduction among infertile couples. To help these individuals generate their own descendants, the ART “intracytoplasmic sperm injection” (ICSI) technique was developed by Dr. Gianpietro Palermo. ICSI allows fertilisation to occur even when the sperm sample is poor in terms of number and/or motility.  The technique uses a micromanipulation station fitted to an inverted microscope equipped with contrast optics that enable three-dimensional visualisation of living cells. The ICSI micromanipulation equipment consists of two glass pipettes; a holding pipette to fix the oocyte and an injection pipette to introduce the sperm into the oocyte cytoplasm (see below video). The ICSI insemination strategy allows fertilisation to occur even under unfavourable conditions, and it can perhaps be hypothesised that this technology may be of assistance in the case of sperm motility deficiencies in outer space, where micro-G conditions may prevent natural, unassisted sperm-egg fusion. Undoubtedly, much more research needs to be performed before we can erase the large question marks that remain as to the likelihood of natural fertilisation taking place in mammals under micro-G conditions, and if required, how effective current assisted reproduction technology would be when using the ART setup and equipment developed on Earth. With talk of future Moon and Mars colonisation and space hotels in the coming decades, there will come a time when human reproduction under microgravity conditions will need to be better addressed if life is to be sustained in off-Earth environments.
Comments are closed.
|
Welcometo the InnovaSpace Knowledge Station Categories
All
|
InnovaSpace Ltd - Registered in England & Wales - No. 11323249
UK Office: 88 Tideslea Path, London, SE280LZ
Privacy Policy I Terms & Conditions
© 2024 InnovaSpace, All Rights Reserved
UK Office: 88 Tideslea Path, London, SE280LZ
Privacy Policy I Terms & Conditions
© 2024 InnovaSpace, All Rights Reserved
 RSS Feed
RSS Feed